Subsurface limestone beds (SLBs) are used as a passive treatment technique to remove toxic metals from acid mine drainage (AMD). In this study, we investigated the mechanisms and thermodynamics of metal (manganese, copper, zinc, cadmium, and lead) precipitation in the SLB installed at the Motokura Mine. Field surveys in 2017 and 2018 showed that the pH of the SLB influent (initially 5–6) increased to approximately 8 in the drain between 24 and 45 m from the inlet. This increase was caused by limestone dissolution and resulted in the precipitation of hydroxides and/or carbonates of copper, zinc, and lead, as expected from theoretical calculations. Manganese and cadmium were removed within a pH range of approximately 7–8, which was lower than the pH at which they normally precipitate as hydroxides (pH 9–10). X-ray absorption near-edge structure analysis of the sediment indicated that δ-MnO2, which has a high cation-exchange capacity, was the predominant tetravalent manganese compound in the SLB rather than trivalent compound (MnOOH). Biological analysis indicates that microorganism activity of the manganese-oxidizing bacteria in the SLB provided an opportunity for δ-MnO2 formation, after which cadmium was removed by surface complexation with MnO2 (≡ MnOH 0 + Cd 2+ ⇄ ≡ MnOCd + + H + ). These findings show that biological agents contributed to the precipitation of manganese and cadmium in the SLB, and suggest that their utilization could enhance the removal performance of the SLB.


Acid mine drainage (AMD) that contains large amounts of toxic metals, particularly manganese (Mn), iron (Fe), copper (Cu), zinc (Zn), arsenic (As), cadmium (Cd), and lead (Pb), is hazardous to the environment around active and abandoned mines. The addition of neutralizing agents, such as slaked and quick limes that form hydroxides, carbonates, surface complexation, and/or coprecipitation with ferrihydrite (Fe(OH)3) and gibbsite (Al(OH)3) 1,2,3 , is used to treat AMD with a high loading of toxic metals, especially Mn, Fe, and aluminum (Al). Passive treatments that use various natural geochemical and biological processes are also used as treatments instead of active treatments. Passive techniques require a certain maintenance cost but little resource input compared with active treatments using neutralizing agents 4,5 . Open limestone channels (OLCs) and limestone leach beds (LLBs) are the simplest construction and operation of the passive treatment systems that use large limestones. The OLCs tend to be constructed in a steep slope area and AMD is neutralized as it passes through the slope. LLBs are small basins and AMD is discharged with upward or downward flow after reaction in the basin 5,6 . These systems require a simple operation and low cost; however, the neutralizing capacity of the OLCs sometimes decreases significantly within a short period; this is because oxic neutralization of high-load AMD causes rapid clogging and coating of the limestone surfaces by hydroxides and carbonates of Fe and Al 5,6,7 . Wetlands are also used to remove metals by biogeochemical reactions involving vegetation, microorganisms, and organic matter in peat moss, spent mushroom compost, sawdust, and straw/manure 5,6,8 . To treat AMD of a high acidity, limestone bed agents are added to the system to supply bicarbonate ions continuously 6 . Limestone bed agents are a simple passive treatment system but it may be difficult to remove Mn from AMD that contains a high amount of Fe; this is because the Mn oxidation rate is slower than Fe and it inhibits or reverses the Mn oxidation 6,9 . Thus, these systems require enough area and residence time for the successful removal of Mn 5,6 .
Subsurface flow systems are developed as a technique to prevent contact of AMD with atmospheric O2. Buried trenches or beds are filled with limestone through which AMD is passed without exposure to the atmosphere and the escape of carbonate dioxide, which results in the promotion of limestone dissolution and an increase in AMD pH and alkalinity 5,6,10 . Anoxic limestone drains (ALDs), through which deoxygenated AMD flows, have been installed to treat AMD at many mine sites in the USA 11,12 and Europe. Although Fe is not precipitated until pH 8 under deoxygenated conditions, excess dissolved Fe, Al, and O2 reduces the treatment performance and lifetime of the ALDs by clogging with their precipitants 6 . Thus, general guidelines recommend AMD introduction with elemental concentrations of below 1 mg L −1 into the ALDs to ensure effective operation 13,14 . Skousen and Ziemkiewicz described the performance of 36 ALDs that were installed in the USA. Most systems with a proposed lifetime of 20 years have been operating for more than the expected lifetime, although some systems lost their neutralizing ability after 8–9 years of operation 15 . The residence time of the ALD systems have shown that the highest removal efficiency was 2–6 h, whereas most systems had residence times between 20 and 100 h 15 . Hedin et al. developed an AMD sizing model based on the capacities of the ALDs installed at 13 coal mines in Pennsylvania, USA 13 . In their model, the minimum ALD size (m 2 ) was determined by dividing the acidity loading (g day −1 ) by 7. This model was based on the difference in chemical composition before and after passage through the ALD but did not consider the specific biochemical reactions that occurred within the ALD 14 . In practice, pH ranges within which each metal is removed by precipitation and adsorption reactions differ depending on the water chemistry. To ensure that passive treatment systems treat AMD efficiently, the process must be scientifically tailored to the water quality and quantity at each mine. Thus, an increased understanding of the specific removal mechanisms and the development of geochemical models, for the different passive treatment options, is essential to choose the sizing processes that are best suited to the water quality and quantity at a particular mine.
A pilot-scale subsurface limestone bed (SLB) has been installed at the Motokura Mine (Hokkaido, Japan) since 2015. Although the SLB removes multiple toxic metals, the biogeochemical mechanisms by which it does so are unclear. In this study, we monitored changes in the drainage and sediment chemistries within the SLB and examined the specific removal mechanisms based on a chemical and biological analysis of the samples and chemical equilibrium calculations.
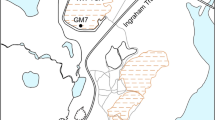
The Motokura Mine is located 23 km southwest of the mouth of the Tokushibetsu River, upstream of a tributary of the Ohun Tarmanai River in Hokkaido, Japan (Fig. 1). The Hokkaido government has been treating AMD at this mine since 1982. AMD that is discharged from the tunnels at the Motokura Mine contained 0.76–12 mg L −1 Fe, < 0.01–0.25 mg L −1 Cu, 0.090–0.52 mg L −1 Pb, 0.62–3.1 mg L −1 Zn, < 0.005–0.011 mg L −1 Cd, and < 0.005–0.28 mg L −1 . The average AMD pH was 4.8–6.8, as measured between 2012 and 2013 16 .
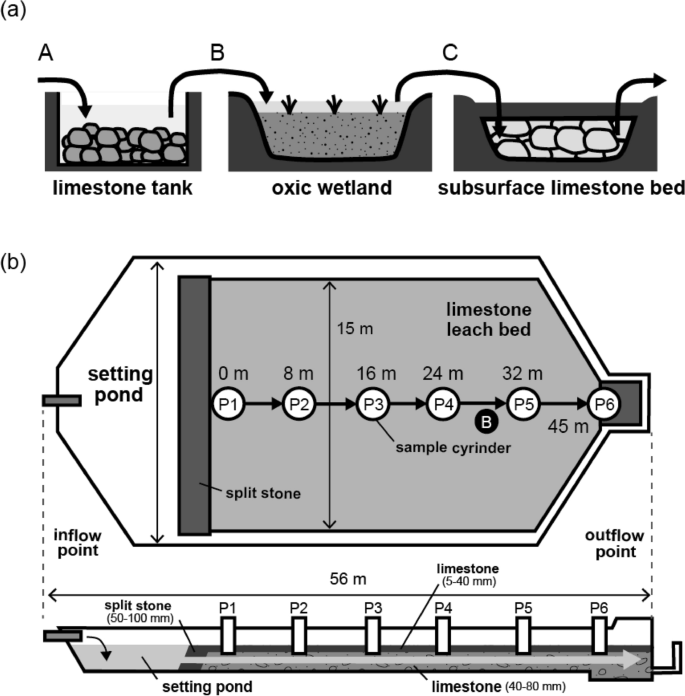
Three passive treatment systems (a limestone tank, an oxic wetland, and a SLB) were installed at the Motokura Mine in 2013 16 . The discharge from six sites (100 m, 70 m, Monju, Sanwa, Heiantsudo tunnels, and the deposit sites) is collected in the catchment basin, from which it flows in series into the limestone tank, oxic wetland, and SLB (Fig. 2a). We collected and monitored the quality and quantity of drainage that entered each system at two field surveys on May 25, 2017 and May 29, 2018. The chemical compositions of the drainage before/after entering each system are shown in Table 1.
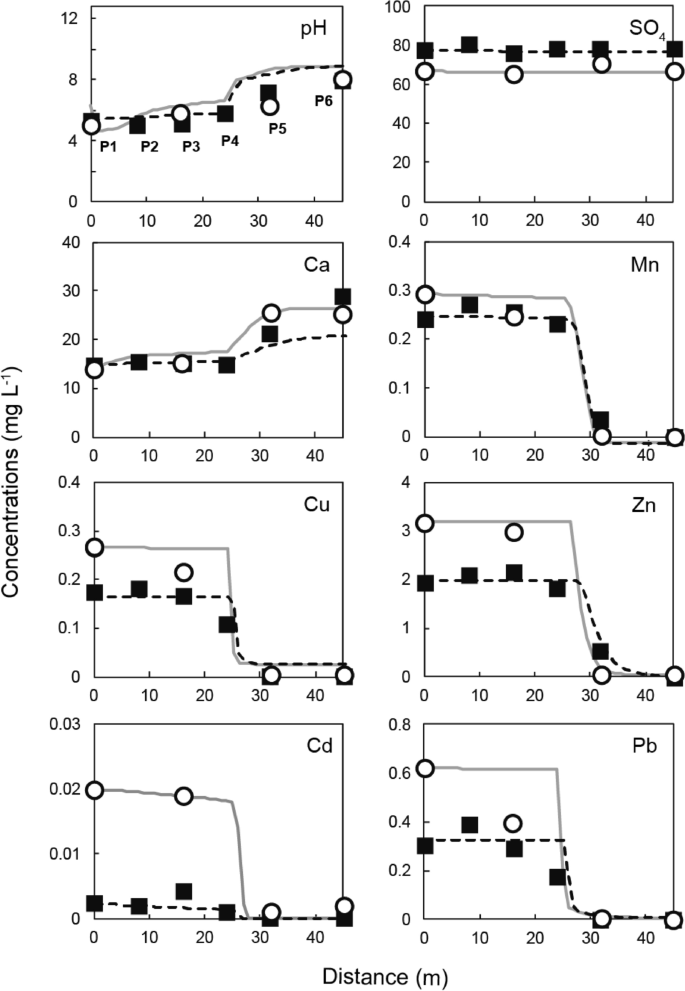
The pH values at the decreasing metal concentrations were 6.3–7.2 (P4–P5 in Fig. 3). Hydroxides of Cu and Pb were calculated to precipitate at a pH close to 6, which agreed with the field observations in the SLB. A higher pH was generally required to remove Mn, Zn, and Cd by hydroxide formation. Mn (II) is oxidized to Mn (III) at a pH of approximately 9, and precipitates as manganese oxyhydroxide (MnOOH) 22 , and Zn and Cd precipitate at pH values of approximately 8 and 9–10, respectively 23 . However, the concentration of Zn, Mn, and Cd decreased at a pH of approximately 7–8, which is lower than the pH at which their hydroxides normally precipitate. This behavior suggests that other factors, such as bioactivity and water chemistry, along with neutralization, contribute substantially to the removal of Mn and Cd in the SLB.
The mineralogy and chemistry of manganese oxide collected from point B of the SLB (shown in Fig. 2a) were analyzed to identify the specific removal mechanism of Mn and Cd from the neutral-pH drainage. Different manganese oxides formed depending on the oxidation state of Mn: γ-MnOOH for Mn (III) and δ-MnO2 for (IV). The type of Mn oxide was determined from the oxidation state obtained by Mn-edge XAFS analysis. The fit of the sample XANES spectra to the reference materials showed that δ-MnO2 was predominantly formed (> 99%) (Fig. 4). In general, the oxidation of Mn(II) to Mn(III) and M(IV) is slow in an abiotic system, with a half-life of a few hundred days or years even under alkaline condition (pH of 8–10 at 25 °C), and the main products are MnOOH (manganite) or other Mn(II)/(III) mixture compounds 22,24 . The MnOOH is thermodynamically metastable and gradually transforms to MnO2, but this reaction depends on ill-defined physicochemical conditions, and often takes months or years 25 . Thus, theoretically, manganite and other low-oxidation-state compounds, such as Mn3O4, should have been found in the SLB when abiotic oxidation was dominant. However, our XAFS analysis showed clearly that the Mn(II) in the AMD was oxidized to Mn(IV), which resulted in the formation of δ-MnO2 in the SLB.
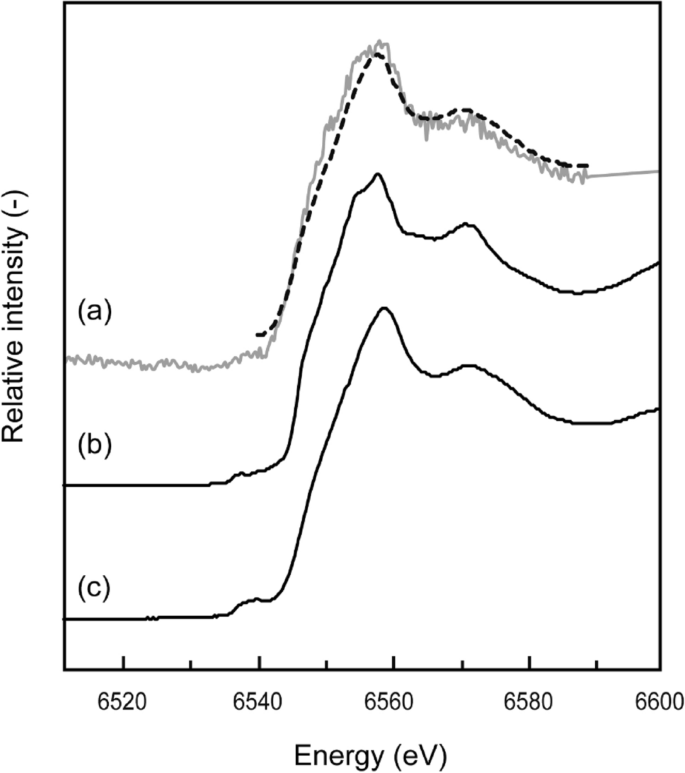
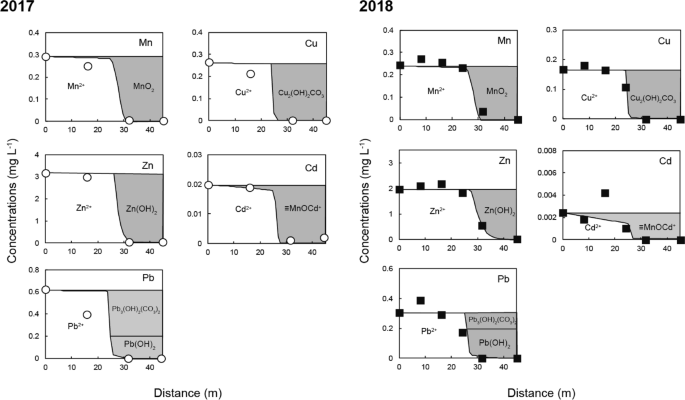
This rapid oxidation of Mn could be attributed to the presence of Mn-oxidizing bacteria in the SLB. Real-time PCR analysis of the 16S rDNA gene of eubacteria collected from the sediment samples indicated the existence of two types of Mn-oxidizing bacteria: Pseudomonas and Bosea 26 . Villalobos et al. reported that Pseudomonas species can produce a layered Mn oxide that is close in structure to δ-MnO2 27 . Furuta et al. explained that MnO2, with an amorphous and fibrous structure from Mn (II) (over 5.5 mg L −1 ), can be synthesized by the activity of Bosea species at neutral pH (6.0–6.3) and under anaerobic (approximately 5–10% oxygen in the gas phase) conditions 28 . Once δ-MnO2 is formed, Mn oxidation is promoted by autocatalytic reaction with adsorption of Mn 2+ on the δ-MnO2 surface. Therefore, δ-MnO2 would be formed initially by the activity of Mn-oxidizing bacteria and gradually accumulated by the combination of microbial and autocatalytic oxidation in the SLB. The ion-exchange capacity of δ-MnO2 (240 meq/100 g) is higher than that of other metal oxides and even clay minerals, such as montmorillonite 29 . Recent studies have revealed that δ-MnO2 can adsorb Cd from solution at pH 6 by surface complexation and coprecipitation 30 . They explained that the removal mechanisms can change depending on the Cd/Mn molar ratio; surface precipitation and/or intercalation can occur when the Cd/Mn molar ratio exceeds 1, whereas simple surface complexation becomes the dominant reaction at a lower Cd/Mn molar ratio (< 1). On the basis of these reports, the Cd/Mn molar ratios in the drainage that were installed in the SLB were low (0.033 in 2017 and 0.0049 in 2018), which indicates that Cd could be removed mainly by surface complexation formation on δ-MnO2 in the SLB. The δ-MnO2, which has a high affinity for metal cations, was most likely formed by Mn-oxidizing bacteria and not by abiotic oxidation, and this plays an important role in efficient removal of Cd from the drainage even at neutral pH.
Changes in the chemical composition of the flow through the SLB were simulated using equilibrium calculations in PHREEQC, including precipitation, surface complexation, Fe and Mn oxidation, and calcite dissolution reactions. The simulation results compared well with the measured values from two field surveys (Fig. 3). The composition at point P1 was used as the initial composition for the calculations (Table 2), and the flux was calculated by dividing the flow rate in the SLB by its cross-sectional area. The actual flow rates in 2017 and 2018 were 0.042 and 0.052 mL min −1 , respectively. The parameters for each kinetic calculation were determined by fitting to the measured values. For the Mn oxidation rate, the calculated rate constants k1 and k2 (see Eq. 2 in the supporting information) were 4.9 × 10 –7 and 2.1 × 10 10 , respectively. The Mn-oxidation rate in the SLB caused by the activity of Mn-oxidizing bacteria was significantly faster than the oxidation caused by atmospheric air. Different microbial species and densities may be present at other field locations. The rate constants for calcite dissolution, k1, k2, and k3, (see Eq. 3 in the supporting information) were those reported by Plummer et al. 21 , and the reactive surface area of calcite exposed to water was calculated by fitting. The exposed calcite reactive surface area decreased gradually along the length of the SLB because of secondary mineral formation caused by the neutralization. Different Ca concentration patterns were found in the upstream (0–24 m) and downstream (25–45 m) portions of the SLB. These regions were treated separately in the calculations (Table 3). The reactive surface area of calcite was 23–33 times smaller in the upstream portion than in the downstream portion, which suggests that the neutralization capacity of the calcite in the upstream portion was diminished by reaction with the drainage. Surface complexation that led to Cd removal in the SLB was controlled by adsorption on the δ-MnO2 surface as explained above. No adsorption equilibrium constant for this reaction has been reported in the literature to the best of our knowledge. We therefore experimentally investigated the adsorption of Cd by δ-MnO2 and found the value of the adsorption equilibrium constant to be Log K = 2.9 (see supporting information for details) (Fig. S3). As shown in Fig. 3, the calculated values for pH and each metal corresponded well with the values measured in the two field surveys.
As shown in Table 3, the estimated reactive surface area of calcite exposed to water was 23–33 times smaller in the upstream portion of the SLB than in the downstream portion. This estimate decreased slightly in 2018 compared with 2017. This means that the neutralization capacity of the calcite was gradually reduced over time because of coating by secondary mineral formation in the SLB. The Ca concentrations in the upstream portion of the SLB were in the same range in 2018 and 2017 (1.4–1.6 mg L −3 ), which indicates that less calcite reacted with AMD. Many studies have investigated the coating of limestone surfaces by the formation of Fe(OH)3, Al(OH)3, and gypsum (CaSO4) by laboratory-scale experiments using calcite-paved columns 32,33 . Soler et al. studied how the neutralization capacity of calcite was reduced by coating. Artificial AMD that contained 250–1500 mg L −1 Fe and 250–1500 mg L −1 SO4 at pH 2 was passed through 1.2–6.0 cm columns packed with limestone particles of approximately 5 mm in size 33 . The reactive surface area of limestone decreased by half over 230 h, and after 300 h it was covered by Fe(OH)3 and CaSO4. In an SLB, the precipitation of metals (Zn, Mn, Cu, and Pb) and CaSO4 could reduce the neutralization capacity of limestone. Periodic dredging is therefore required to maintain the treatment capacity of an SLB. For the SLB in the Motokura Mine, the rate at which the SLB loses its neutralization capacity has not yet been determined because the limestone in SLB was not dredged since its installation in 2013. The secondary mineral formation on calcite would provide a reason for the low calcite dissolution in the SLB for our field surveys in 2017 and 2018.
The removal of metals (Mn, Cu, Zn, Cd, and Pb) by an SLB that was installed at the Motokura Mine was evaluated by field analysis and chemical equilibrium calculations. The pH of drainages that were introduced to the SLB (initially 5–6) increased to approximately 8 between 24 and 45 m from the SLB inlet. This was caused by limestone dissolution, and was followed by the precipitation of Cu, Zn, and Pb as hydroxides and/or carbonates. The experimental results matched the thermodynamic calculations well. Mn and Cd were removed in a pH range of approximately 7–8, which is lower than the pH at which they normally precipitate as hydroxides (pH 9–10). The removal occurred because the formation of δ-MnO2 was accelerated in the SLB and surface complexation of Cd occurred with its surface. Chemical and biological analysis of the samples and adsorption experiments confirmed the results. Therefore, Mn-oxidizing bacteria and δ-MnO2 production contribute to the efficient removal of Mn and Cd at neutral pH without excessive alkalization in a pilot-scale SLB. The chemical equilibrium calculations, which include the geobiochemical reactions, indicate that 15.5–18.3 h of residence time is required to meet Japanese effluent standards for the 670-m 3 SLB at the Motokura Mine. Geochemical mechanisms and calculations, such as in this work, will be useful for determining a priori whether to install SLBs in mines with different quantities and qualities of AMD.
The synchrotron radiation experiments were performed using the BL5S1 beamline courtesy of the Aichi Synchrotron Radiation Center, Aichi Science & Technology Foundation, Aichi, Japan (Proposal No. 201705007 and 201704009), and the BL14B2 beamline of SPring-8, with approval of the Japan Synchrotron Radiation Research Institute (Proposal No. 2018A1696 and 2019B1867). Part of this work was performed within the activities of the Research Institute of the Sustainable Future Society, Research Institute for Science and Engineering, Waseda University. The authors thank the Japan Oil, Gas and Metals National Corporation (JOGMEC) for their cooperation. We thank Laura Kuhar, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.